lunes, 3 de noviembre de 2008
martes, 21 de octubre de 2008
Bioabsorbable staples
Publish date: Oct 1, 2008
The INSORB/25 skin stapler offers the speed of metal staplers with the cosmetic results and comfort of absorbable sutures, according to Incisive Surgical, Inc. (Minneapolis, MN). The device uses bioabsorbable staples that are placed under the skin and dissolve in the body within a few months. Made of the same material as leading bioabsorbable sutures, the staples break down naturally, so there's no need for postop removal. This product also eliminates the potential for needlesticks to the OR staff. For more information, contact info@insorb.comor visit http://www.insorb.com/ .
domingo, 19 de octubre de 2008
viernes, 17 de octubre de 2008
SÍNTOMAS DE LA MENOPAUSIA: CALORONES
Aunque la mayoría de las mujeres experimentan una reducción de su VMSs por 5 años después del final de la menstruación, las mujeres con a menopausia quirúrgica pueden tener más síntomas persistentes, y hasta al 16% las mujeres con menopausia natural siguen sufriendo
VMSs. Aunque la terapia estrógenica es conocida como una terapia eficaz, no es ni adecuado ni conveniente para cada mujer sintomática. El tratamiento no-hormonal y tratamientos tales como la gabapentina, inhibidores selectivos de la recaptación de serotonina / inhibidores selectivo noradrenalina de la recaptación de drogas, y los antihipertensivos clonidina y alphamethyldopa puede ser útil para algunas mujeres. Cada vez hay más pruebas en apoyo de la ineficacia de muchas propuestas complementarias y otras modalidades. Esta revisión se hará hincapié en la historia natural de VMS y las pruebas que apoyan la evidencia de diversos tratamientos.
CLINICAL OBSTETRICS AND GYNECOLOGY
Volume 51, Number 3, 539–548
r 2008, Lippincott Williams & Wilkins
DESCARGA
domingo, 5 de octubre de 2008
MANEJO DE LA HEMORRAGIA UTERINA DISFUNCIONAL
que ginecólogos y médicos de atención primaria enfrentan en su consultorio.
''Sangrado uterino disfuncional''es el término aplicado a los patrones anormales
de sangrado que se producen en las mujeres secundario a anovulación u oligoovulación
y es a menudo citado como el sangrado anovulatorio. La exclusión de
patología anatómica y enfermedad médica es importante antes de la aplicación de la presente
clasificación. El sangrado es no cíclico que puede ir desde un sangrado escaso a un exceso
en el volumen. El Sangrado disfuncional puede, en casi todos los casos, ser tratada
médicamente para revertir las anomalías del endometrio que conducen a un
flujo menstrual prolongado y, posteriormente, restaurar la previsibilidad y la
regularidad del ciclo.
Management of DysfunctionalUterine Bleeding. Obstet Gynecol Clin N Am
35 (2008) 219–234
DESCARGA
ARTÍCULO
domingo, 21 de septiembre de 2008
EPISIOTOMÍA: ENCUESTA
Existen evidencias suficientes para no realizar de rutina la episiotomía, ni aún en primigestas. Las evidencias lo demuestran sustentadas en múltiples trabajos científicos. No hay que resistirse a los cambios y nuevos conocimientos.
Resultados de la encuesta: 16 votos en total distribuidos de la siguiente manera:
Núliparas 8 (50%) Múltiparas 0 (0%) Partos pretérminos 6 (37%) Segundo parto 0 (0%) Parto podálica 8 (50%) Parto instrumental 8 (50%).
domingo, 14 de septiembre de 2008
GARDASIL

Gardasil Approval Expanded to Prevention of HPV-Related Vulvar, Vaginal Cancer
“There is now strong evidence showing that this vaccine can help prevent vulvar and vaginal cancers due to the same viruses for which it also helps protect against cervical cancer,” Jesse L. Goodman, MD, MPH, director of the FDA’s Center for Biologics Evaluation and Research, said in a news release. “While vulvar and vaginal cancers are rare, the opportunity to help prevent them is potentially an important additional benefit from immunization against HPV.”
In 2006, the original FDA approval for Gardasil was for the prevention of cervical cancer caused by HPV types 16 and 18, which cause 70% of cervical cancers, and which are implicated in unknown percentages of vulvar and vaginal cancers. The original approval, which was for girls and women aged 9 to 26 years, was also for the indications of preventing precancerous genital lesions caused by HPV types 6, 11, 16, and 18 and genital warts caused by HPV types 6 and 11.
In the United States, HPV is the most prevalent sexually transmitted disease, with an annual incidence of 6.2 million new infections, according to the US Centers for Disease Control and Prevention.
Merck & Co Inc, the manufacturer of Gardasil, followed more than 15,000 participants from the original cervical cancer prevention studies for 2 more years to determine the effects of Gardasil on the risk for vulvar and vaginal cancer compared with control participants who had not received Gardasil.
This follow-up showed that Gardasil was highly effective in preventing HPV-related precancerous vulvar and vaginal lesions among women who tested negative for HPV types 16 or 18 at study enrollment. None of the participants in the Gardasil group developed HPV type 16- or 18-related precancerous lesions compared with control group findings of 10 precancerous vulvar lesions and 9 precancerous vaginal lesions related to HPV types 16 or 18.
Women previously infected with HPV types 16 or 18 before immunization had no evidence of benefit. To optimize the preventive effects of Gardasil, the investigators therefore recommend vaccination before potential exposure to HPV types 16 or 18.
Caveats from the FDA represented in the label are that presently available information is insufficient to support use beyond age 26 years, and that Gardasil does not protect against diseases caused by other HPV types. Because Gardasil does not protect against preexisting HPV infections and because no vaccine is 100% effective, all women should continue to be monitored with Pap tests, even after vaccination.
Most adverse events of Gardasil reported since FDA approval in 2006 have not been serious. The most frequently reported adverse events have included syncope, injection site pain, headache, nausea, and fever. Observation is recommended after vaccination in case of syncope or severe allergic reactions.
A short- and long-term safety surveillance study is underway of 44,000 individuals in a managed care organization who received Gardasil for all its approved uses.
miércoles, 10 de septiembre de 2008
URGENCIA EN OBSTÉTRICIA Y GINECOLOGÍA

DESCARGA
domingo, 7 de septiembre de 2008
sábado, 6 de septiembre de 2008
EPISIOTOMÍA: ¿ESTÁ JUSTIFICADA SU PRÁCTICA EN LOS ACTUALES MOMENTOS?

Hoy en día todavía en las escuelas de medicinas y postgrado de obstetricias, se sigue enseñando de forma férrea la práctica de la episiotomía. Un procedimiento quirúrgico nombrado por primera vez en el año 1742, popularizado y expandido a principio del siglo 20. A veces practicamos procedimientos y conductas médicas sin el basamento de las evidencias y otras veces aún teniendo las evidencias las seguimos practicando, solo por el hecho de resistirnos al cambio.
Según Mardsen Wagner, Ex Director del Departamento de Salud Materno-Infantil de la OMS "la episiotomía nunca es necesaria en más del 20% de los partos. La ciencia ha constatado que causa dolor, aumenta el sangrado y causa más disfunciones sexuales a largo plazo. Por todas estas razones, realizar demasiadas episiotomías ha sido correctamente etiquetado como una forma de mutilación genital en la mujer. El índice de episiotomías del 89% en España constituye un escándalo y una tragedia"
Les traigo estos enlaces para luego saber sus comentarios al respecto.
http://www.episiotomia.info/index.php?option=com_content&task=view&id=30&Itemid=9"
http://www.espacioblog.com/porunpartorespetado/post/2008/09/03/episiotomias-o-desgarros#c3498975
http://www.bmj.com/cgi/reprint/324/7343/945
jueves, 4 de septiembre de 2008
¿Por qué siguen muriendo tantas mujeres durante el embarazo y el parto?
P: ¿Por qué siguen muriendo tantas mujeres durante el embarazo y el parto?
R: Cada minuto, no menos de una mujer muere como consecuencia de complicaciones relacionadas con el embarazo y el parto; es decir, unas 529 000 mujeres cada año. Además, por cada mujer que muere al dar a luz, otras 20 sufren lesiones, infecciones o enfermedades (unos 10 millones de mujeres cada año).
Cinco complicaciones directamente relacionadas son responsables de más del 70% de las muertes maternas: hemorragias (25%), infecciones (15%), abortos peligrosos (13%), eclampsia (hipertensión arterial pronunciada que provoca convulsiones - 12%), y parto obstruido (8%). Si bien esas son las causas principales de mortalidad materna, la falta de cuidados o de acceso a los mismos, su elevado costo o su escasa calidad, son elementos determinantes. Malogran el desarrollo y el bienestar social, y cada año dejan a un millón de niños huérfanos de madre. Esos niños tienen 10 veces más probabilidades de morir durante los dos años siguientes a la muerte de sus madres que los demás.
No hay razón para que las mujeres tengan que morir en el parto. Hay que proporcionar a las mujeres jóvenes la información y el apoyo que necesitan para controlar su salud reproductiva, hay que prestarles apoyo durante el embarazo, y proporcionarles cuidados, a ellas y a sus retoños, hasta que esté bien avanzada la niñez. La inmensa mayoría de las muertes maternas se evitarían si las mujeres tuvieran acceso a servicios de planificación familiar de calidad, atención competente durante el embarazo, el parto y el primer mes después del alumbramiento, o servicios de atención postaborto y, donde esté permitido, servicios de aborto seguros. Un 15% de los embarazos y los partos necesitan cuidados obstétricos de urgencia debido a riesgos difíciles de predecir. Un sistema de salud dotado de personal especializado es decisivo para salvar la vida de esas mujeres.
La OMS se ha comprometido a lograr el Objetivo de Desarrollo del Milenio consistente en reducir en tres cuartas partes la mortalidad materna. Este año, con ocasión del Día Mundial de la Salud, que se celebra el 7 de abril, se quiere sensibilizar a la población acerca de la salud de la madre, del recién nacido y del niño, y señalar como prioritarias esas cuestiones para los gobiernos y la comunidad internacional.
En el Informe sobre la salud en el mundo - ¡Cada madre y cada niño contarán!, presentado en el Día Mundial de la Salud, se pide que mejore el acceso a una atención y unos cuidados que pueden salvar la vida. Se aboga asimismo por un planteamiento que proporcione cuidados sin solución de continuidad a las madres y sus hijos desde antes del embarazo hasta el nacimiento y la infancia del bebé.
Cinco factores causan más del 70% de las muertes maternas. DIEZ DATOS SOBRE SALUD MATERNA
 |
Se trata de:
- hemorragias graves,
- infecciones,
- abortos peligrosos,
- trastornos hipertensivos,
(preeclampsia y eclampsia)
- y parto obstruido.
domingo, 31 de agosto de 2008
sábado, 30 de agosto de 2008
jueves, 28 de agosto de 2008
EMBARAZO POSTERIOR A ESTERILIZACIÓN CON ESSURE
BACKGROUND: Introduced to the U.S. market in late 2002 as a permanent method of contraception, a microinsert device is placed hysteroscopically into the fallopian tubes, not requiring incisions or general anesthesia. This report describes a case of pregnancy more than 6 months after a hysterosalpingogram (HSG) confirming bilateral occlusion after microinsert sterilization.
CASE: A 30-year-old gravida 1 para 1 woman desired permanent sterilization. The patient underwent microinsert device placement and 6 months later had an HSG that confirmed bilateral tubal occlusion. More than 6 months after the confirmatory HSG, the patient became pregnant and delivered a term infant by cesarean birth. Cornual perforation was noted at surgery.
CONCLUSION: This case illustrates pregnancy after microinsertion sterilization and an HSG confirming bilateral tubal occlusion, despite perforation. A microinsert device continues to be a viable option for sterilization.

A 30 year old gravida 1 para 1 morbidly obese woman presented to our clinic desiring permanent sterilization. The patient’s surgical history was significant for a cesarean delivery at term secondary to failure to progress. Her postoperative course was complicated by pulmonary edema and a wound infection requiring long-term wound vacuum placement. After being presented with several options for permanent sterilization, the patient decided to proceed with microinsert device placement.
The patient underwent the microinsert sterilization without complication. Findings at the time of hysteroscopy included a normal endometrial cavity and normal tubal ostia bilaterally. Expanded coils were trailing into the uterine cavity bilaterally after placement. Before discharge from the hospital, the patient received depomedroxyprogesterone acetate for contraception.
The patient was seen for her 6-week postoperative visit and was without complaints. A 3-month HSG was scheduled. The patient presented as scheduled for her HSG, which was performed using CooperSurgical (Trumbull, CT) surgical hysterosonography and hysterosalpingography catheter with a 1.5-mL balloon. Twenty milliliters of sonografin 38% organically bound iodine (diatrizoate meglumine and iodipamide meglumine) contrast media was used. The report was read as right tubal occlusion and left tubal patency with spillage of contrast into the peritoneal cavity. The patient was seen again in clinic, and the results were explained to her. Per the microinsert company’s guidelines, depomedroxyprogesterone was continued, and a repeat HSG was performed 3 months later. The interpretation was bilateral tubal occlusion (Fig. 1). A nonsteroidal anti-inflammatory drug was not given at the time of either HSG.
More than 6 months later, the patient missed her menstrual cycle and performed a pregnancy test at home, and the result was positive. The patient presented to the emergency department because of this, and a sonogram was performed. The patient was noted to have an intrauterine pregnancy a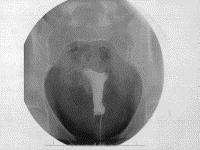 t 7 weeks of gestation with fetal cardiac activity.
t 7 weeks of gestation with fetal cardiac activity.
The patient had a routine prenatal course. The patient presented in active labor and, given her history of prior cesarean delivery, desired a repeat cesarean delivery, with a bilateral tubal ligation. At the time of surgery, the microinsert was found to have perforated the uterus in the left cornual area. A tubal ligation was then performed.
COMMENT
This case report is one of only two found reporting pregnancy after microinsert hysteroscopic sterilization and bilateral occlusion confirmed by HSG. We searched Ovid/Medline and PubMed using the following search terms in English: “microinsert device,” “permanent birth control,” “hysteroscopic sterilization,” and “conceptus.” Between January 2000 and July 2007, there is only one other case reporting pregnancy after microinsert device tubal sterilization with confirmed occlusion on HSG. This study by Moses et al 9 reports a patient who became pregnant despite an HSG showing tubal occlusion. This patient was ultimately found to have a microinsert perforating the uterine wall. There is another case report of failed tubal occlusion using the microinsert device permanent birth control hysteroscopic sterilization procedure; however, that report resulted in a patent tube by HSG, but pregnancy did not occur.6 In a reported case from Australia, the device was appropriately placed and retained; however, the device never occluded the right fallopian tube. In a follow-up editorial, the author questions the interpretation of the follow up HSG and plain film x-ray.7 The author states that the microinsert was lodged above the wall of the fallopian tube, most likely subserosal, and that two tracts can be seen on the x-ray.
Upon review of the early studies and phase II and III trials, complications were very limited. These included inability to place the device bilaterally due to anatomic, procedural, and device-related events, uterine wall or tubal perforations, some of which were thought to be due to a support catheter that has since been discontinued, and microinsert expulsion.2,3,4 In an early study, the device was placed bilaterally in 85% of women with no pregnancies reported, and the procedure was tolerated very well.4 In phase II trials, bilateral placement was successful in 88% of cases.3 There were no pregnancies reported in this trial. There was unsatisfactory placement in 4% of cases including one microinsert expulsion, six perforations, and two unsatisfactorily placed devices. At 3-month HSG, 96% showed bilateral occlusion, 3% had unilateral occlusion, and 1.5% had an “equivocal” HSG. At 6 months postprocedure HSG, all women who had at least one patent fallopian tube at the 3-month HSG showed bilateral occlusion.
During the phase III study, bilateral placement was achieved in 92% of cases.2 Of those with bilateral placement, HSG at 3 months showed bilateral occlusion in 92% of women. As in the phase II study, all women who did not have occlusion at 3 months had a 6-month postprocedure HSG, which showed bilateral occlusion. Complications included a 3% expulsion rate, a 0.9% perforation rate, and an unsatisfactory placement rate of 0.6%. Including all phases as of January 8, 2003, there were no pregnancies after 9,620 woman-months of exposure.2 In our case, the HSG was interpreted as showing bilateral tubal occlusion. The patient then became pregnant 6 months after the confirmatory HSG, nearly 1 year after her initial procedure. Some hypotheses as to how this could occur are suggested.
The first is that there was unsatisfactory placement with only two trailing coils into the uterine cavity. Ideally, there should be three to eight expanded coils. The microinsert device company recommends if there are fewer than 3 or more than 8 coils seen that the device should be left in place and the patient should be evaluated as planned by HSG at 3 months postprocedure. Our case followed this recommendation, and the device showed bilateral tubal occlusion at 6 months.
Another possibility is that at the time of HSG, tubal spasm occurred causing a false evaluation of tubal occlusion. An important consideration might be to give a nonsteroidal anti-inflammatory drug 30–60 minutes before the HSG, as is done at the time of the procedure. Another way to reduce tubal spasm is to slowly inject the contrast at the time of HSG. This, however, was not done during this case. Another theoretical explanation for pregnancy after microinsert device could be assisted reproductive techniques; this, however, was not the case in this patient. Last, as in the case by Moses et al 9 uterine perforation by the microinsert device in the proximity of the tubal ostia may mimic proper microinsert placement and bilateral tubal occlusion. As we later found out, this was the case in this patient.
This case illustrates pregnancy after microinsert device hysteroscopic sterilization and an HSG showing bilateral occlusion. As of the end of July 2007, the microinsert device website reports 130,000 procedures have been done. Our case and the case by Moses et al 9 are the only two that can be found, using our search criteria, that have had microinsert device with bilateral occlusion on HSG and a subsequent pregnancy. We now know that, as in the other case by Moses et al,9 there was a uterine perforation. In the context of knowing all sterilization procedures have a failure rate, a microinsert device continues to be a viable option for permanent sterilization. We continue to offer this procedure to our patients. We contend, however, that as with all medical procedures, failures do occur.







